Updates in Pancreatitis: Innovation and PANOQUELL®-CA1 (Fuzapladib Sodium for Injection)
Pathogenesis1
Pancreatitis is a common disease in dogs – more common than we recognize! The reported prevalence varies, depending on the study, causes are likely not known, and the clinical signs observed have a wide range from mild to severe and can escalate rapidly.
Although most cases in dogs are reported as idiopathic, there is limited research regarding etiology. While additional research is needed to further define underlying causes, predisposing factors such as garbage ingestion and breed predisposition are well recognized.
The hallmark of acute pancreatitis (AP) in dogs is neutrophilic inflammation within the tissues of the pancreas. But what causes it? The historical premise that the pathogenesis is exclusively based on ‘pancreatic autodigestion’ has evolved. While various insults can potentially lead to premature activation of zymogens, which in turn causes production of cytokines leading to inflammation, other cases may not be associated with premature activation of zymogens and autodigestion. Continued understanding in this area leads to improved treatments, which until now, has been centered around supportive care. It is important to note that all humans with pancreatitis experience pain as a symptom and dogs are likely no different. Even without obvious clinical signs consistent with pain, it’s reasonable to assume if the dog has pancreatitis, the dog is experiencing pain.
Diagnosis2
Once the clinician evaluates the history and physical exam, if pancreatitis is suspected, a CBC, biochemical profile, and urinalysis are warranted. Currently, serum pancreatic lipase immunoreactivity (cPLI as measured by Spec cPL) concentration is the ‘test of choice’ for diagnosis of pancreatitis. While commonly thought to be the gold standard for diagnosis, pancreatic histopathology samples may not be uniformly representative in all cases and can be impractical in the compromised patient.
Inflammation and pancreatitis3,4
AP is associated with neutrophilic inflammation. The process of extravasation of neutrophils into the tissue and associated tissue damage, is dependent on a very specific protein-protein interaction between the neutrophil and the vascular endothelial surface. The critical players in this interaction are the protein, Leukocyte Function Associated Antigen-1 (LFA-1), and its ligand, Intercellular Adhesion Molecule-1 (ICAM-1). LFA-1 is expressed on the neutrophil surface and ICAM-1 is expressed on the vascular endothelium. This interaction is required for the neutrophilic extravasation that occurs in AP. LFA-1 is activated by chemokines, and ICAM-1 is upregulated, leading to the ‘arrest of circulating neutrophils at the site of inflammation. Once in the tissue, neutrophils release additional inflammatory mediators, which attract additional neutrophils and inflammatory cells, leading to tissue damage and potentially clinical complications.
In severe cases, extra-pancreatic inflammation, potentially leading to multi-organ failure, systemic inflammatory response syndrome (SIRS), and death can occur.
Current Standard of Care5
Until now, the current standard of care for canine pancreatitis management has been supportive care only. These clinical interventions include:
- Fluid therapy
- Antiemetic therapy (maropitant, ondansetron, or combination)
- Pain management (meperidine, butorphanol, buprenorphine or continuous rate infusion (CRI) with morphine, fentanyl, methadone or other)
- Nutritional support
- Management of Underlying Causes and Risk Factors
- Management of risk factors, including hypertriglyceridemia, and hypercalcemia
- Management of concurrent diseases, such as hepatitis, IBD, and diabetes mellitus
- Management of systemic complications
PANOQUELL®-CA1 – an LFA-1 activation inhibitor1,3,4
As noted in the diagram below and discussed above, the process of extravasation of neutrophils in AP involves activation of LFA-1 (on the neutrophil surface) and upregulation of its ligand ICAM-1 (on the endothelial wall). Fuzapladib is an inhibitor of LFA-1 activation.
PANOQUELL®-CA1(fuzapladib sodium for injection) is the first conditionally approved drug targeting acute pancreatitis in dogs by blocking LFA-1 mediated inflammation. Inhibition of LFA-1 activation results in:
- Reduced potential for extra-pancreatis inflammation, SIRS, and multi-organ failure.
- Reduced neutrophilic infiltration into the pancreas.
- Reduced pancreatic inflammation, supporting faster recovery.
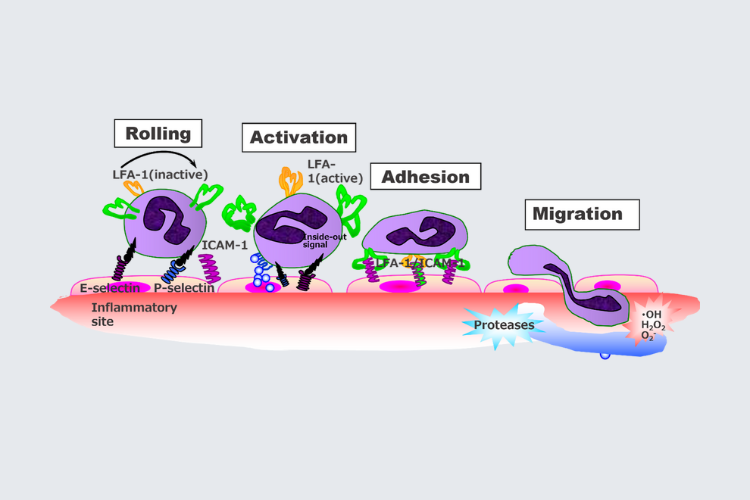
Neutrophil extravasation into the pancreas and therapeutic manipulation with fuzapladib sodium. Fuzapladib sodium inhibits leukocyte function antigen‐1, which prevents neutrophils from migrating from capillaries into the surrounding tissue.
ICAM‐1(Intercellular Adhesion Molecule 1), immunoglobulin‐like cell adhesion molecule 1; LFA‐1, leukocyte function antigen‐11
PANOQUELL®-CA1 (fuzapladib sodium for injection)
Introducing the first FDA conditionally approved treatment for acute canine pancreatitis.
The primary studies to support conditional approval are a 3-day placebo-controlled clinical trial and a safety study.
3-day placebo-controlled clinical trial
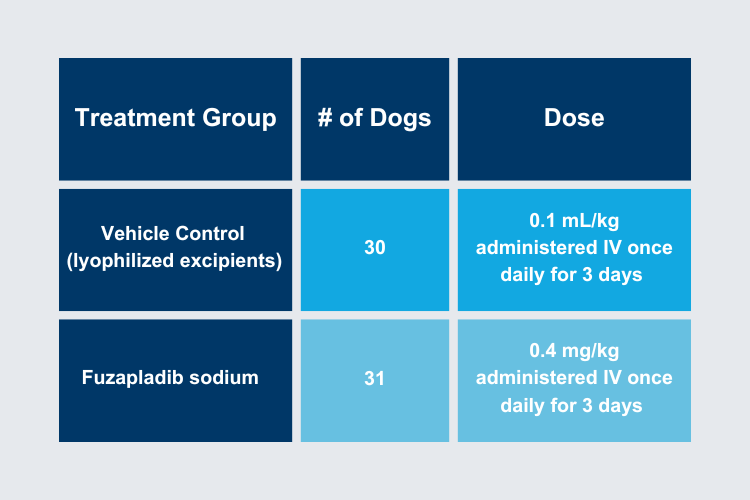
This study evaluated 36 dogs over a 3-day period from 11 US study sites. The primary inclusion criteria included > 2 clinical signs of acute pancreatitis, and Day 0 abnormal SNAP-cPL® and a Spec cPL® value ≥ 400 μg/L (IDEXX Laboratories, Westbrook ME).
A Modified Canine Activity Index (MCAI (7)) was used to evaluate and score the following clinical signs: activity, appetite, vomiting, cranial abdominal pain, dehydration, stool consistency, and blood in the stool.
The primary effectiveness variable was the change in the group mean total MCAI(7) score from Day 0 (pre-treatment) to Day 3, as assessed by the Investigator.
Treatment:
Fuzapladib sodium Group
0.4 mg/kg fuzapladib sodium
IV once daily for 3 days with Standard of Care (SoC) as a bolus over 15 seconds to 1 minute
Vehicle control group
0.1 mL/kg vehicle control
IV once daily for 3 consecutive days with SoC
SoC for Both Groups
ALL dogs received SoC for pancreatitis, including one or more of the following: fluids, nutritional support, pain medications (excluding NSAIDS), anti-emetics, and/or medications used to treat well-controlled pre-existing conditions.
Results:
- Day 0: Mean scores for fuzapladib sodium group and vehicle control group were similar (i.e., 8.53 vs. 7.68).
- Mean change of the MCAI (7) score from D0 to D3 for fuzapladib sodium group and vehicle control group was -7.7 and -5.7.
- Fuzapladib sodium group demonstrated a statistically significant reduction in MCAI (7) score (p=0.0193), compared to the vehicle control group.
Clinical Safety Adverse Reactions (Top 8 AE’s listed):
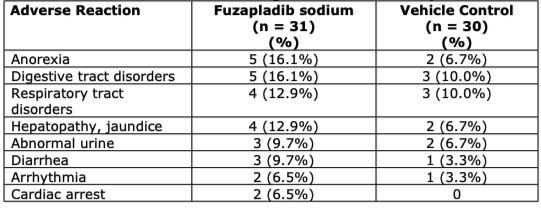
Additionally, 5 deaths/euthanasia’s were present
- 4 in the fuzapladib sodium and 1 in the vehicle control group.
- 2 additional vehicle control group dogs were euthanized shortly after completion of the study.
Conclusion:
The study results support a reasonable expectation of effectiveness for the use of PANOQUELL®-CA1. It is administered IV at the conditional dose of 0.4 mg/kg once daily for three days for the management of clinical signs associated with acute onset of pancreatitis in dogs.
Target Animal Safety Study
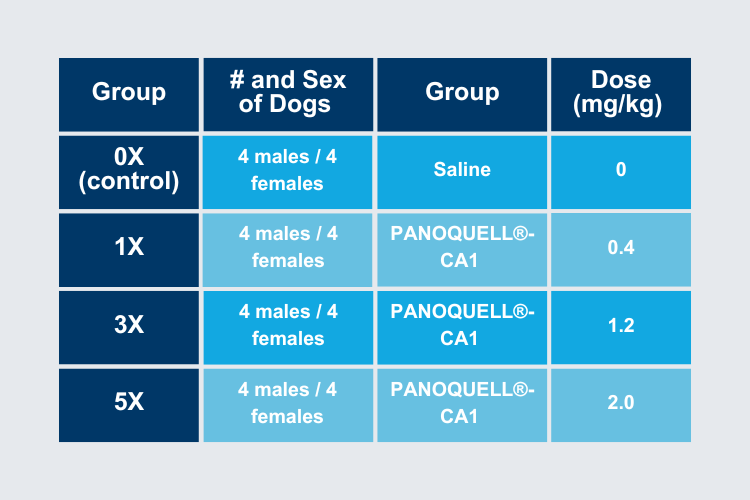
32 Healthy dogs
- All intact
- 6-7 months of age
- 5.8 – 8.7 kg body weight
4 groups which received IV injection every 24 hours for 9 days
- Saline Control
- PANOQUELL®-CA1
- 0.4 mg/kg (1X dose)
- 1.2 mg/kg (3X dose)
- 2.0 mg/kg (5X dose)
Conclusion:
- The administration of PANOQUELL®-CA1 as an IV injection once daily for nine days at doses of 0, 0.4 mg/kg (1x dose), 1.2 mg/kg (3x dose) , or 2 mg/kg (5x dose) fuzapladib sodium did not produce systemic toxicity and had an acceptable margin of safety.
- The administration of PANOQUELL®-CA1 resulted in swelling and bruising at the injection site, with associated gross pathology and histopathological findings, hypertension, and mild thrombocytopenia.
- This nine-day safety study supports the safe use of PANOQUELL®-CA1 when administered IV to dogs, according to the label.
Conditional Approval Eligibility7
- The new animal drug is intended to treat a serious or life-threatening disease or condition OR addresses an unmet animal or human health need; AND
- A demonstration of effectiveness would require a complex or particularly difficult study or studies.
PANOQUELL®-CA1 was determined to be eligible for conditional approval under these provisions because it controls a serious or life-threatening disease or condition, addresses an unmet animal health need, and the demonstration of effectiveness requires a complex or particularly difficult study or studies.
Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-567.
It is a violation of Federal law to use this product other than as directed in the labeling.
References
- Cridge, H. et al. New insights into the etiology, risk factors, and pathogenesis of pancreatitis in dogs: Potential impacts on clinical practice. J Vet Intern Med. 2022; 36(3): 847-864. Doi:10.1111/jvim.16437
- Xenoulis, P.G. Diagnosis of pancreatitis in Dogs and Cats. Journal of Small Animal Practice (2015) 56, 13–26 DOI: 10.1111/jsap.12274
- Thorlacius, H., et al. (2011), Lymphocyte function antigen-1 regulates neutrophil recruitment and tissue damage in acute pancreatitis. British Journal of Pharmacology, 163: 413-423. https://doi.org/10.1111/j.1476-5381.2011.01225.x
- Sun W, Watanabe Y, Wang ZQ. Expression and significance of ICAM-1 and its counter receptors LFA-1 and Mac-1 in experimental acute pancreatitis of rats. World Journal of Gastroenterology. 2006 Aug;12(31):5005-5009. DOI: 10.3748/wjg.v12.i31.5005. PMID: 16937496; PMCID: PMC4087403.
- Merck Manual (online), The Exocrine Pancreas of Dogs and Cats, Joerg Steiner, Oct 2020.
- PANOQUELL®-CA1 (fuzapladib sodium for injection) Product Insert https://www.cevaconnect.com/internal-medicine/library/
- Freedom of Information Summary. PANOQUELL®-CA1 FOI Summary for the Conditional Approval of 141-567 November 14, 2022 (fda.gov)
PANOQUELL®-CA1 is a registered trademark of Ishihara Sangyo Kaisha, Ltd. Learn more and shop for this product on our website, here.